Abstract
Immune checkpoint inhibitors (ICI's) have revolutionized the management of patients with advanced melanoma (AM). However, the data on the effectiveness of ICI's has been largely restricted to patients on clinical trials, thus excluding patients with second malignancies. Chronic Lymphocytic Leukemia (CLL) is the most prevalent adult leukemia and is associated immune dysregulation and a 3.8 fold increase in melanoma incidence. We report a multi-center, retrospective case-control study examining differences between patients who received checkpoint therapy in the context of the dual diagnoses of CLL and advanced melanoma (n = 20) compared to those with advanced melanoma alone (n = 154).
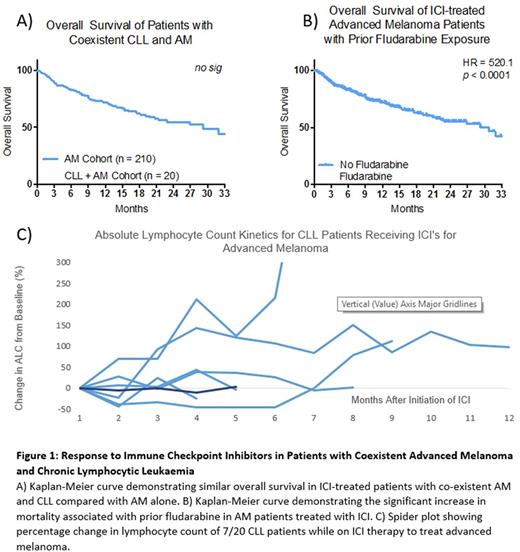
Prognostic features for AM (including age, brain metastasis, visceral involvement, LDH and neutrophil count) were comparable between the two cohorts, however absolute lymphocyte count (ALC) was higher in CLL patients (p <0.0001) as anticipated. 3/20 of the CLL cohort experienced haematological autoimmune adverse events (AIHA, ITP, HLH), which necessitated cessation of ICI therapy; these events were not reported in the non-CLL cohort.
There was not a significant difference between response rates to ICI's, 1-year survival or overall survival between CLL and non-CLL cohorts (Fig 1A). All recorded mortality (8/8) in the CLL cohort was attributable to AM progression or complications of ICI therapy rather than progressive CLL. The most significant predictor of survival in CLL cohort was prior exposure to fludarabine which showed a significantly shorter OS (median 2.3 vs 29.23 months, HR 7.406, p < 0.0001) than those who had not (Fig 1B).
This cohort also offers a unique opportunity to observe the effects of ICI therapy on the progression of CLL. To our knowledge, this is the largest reported cohort of CLL patients treated with checkpoint inhibitors to date. The diagnosis of CLL pre-dated the diagnosis of AM in 18/20 cases. 5 cases had required prior treatment for CLL, while the remaining 15 cases were under observation alone. All 20 cases were Stage 0-1 according to the Rai classification at the time of melanoma diagnosis and no cases demonstrated a transformation to an aggressive subtype. 0/20 cases experienced a haematological response of their CLL while on ICI therapy and 6/20 cases progressed clinically either on the basis of tumour burden or absolute lymphocyte count within 12 months of ICI therapy (Fig 1C).
This retrospective data demonstrates that ICI's have equivalent therapeutic efficacy in AM in patient with concurrent CLL This was unexpected as it was anticipated that the immune dysregulation associated with CLL would impair the ICI-mediated anti melanoma immunological response. The exception to this trend, was the poor efficacy of ICI's in patients with prior fludarabine exposure, presumably owing to the profound T cell depletion associated with this therapy. Additionally, ICI therapy alone appears ineffective in the management of CLL in keeping with recent phase II trial data.
Gill: Janssen: Consultancy, Honoraria, Other: travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal